Thetumor suppressor TP53 is among the most mutated genes in cancer, including many hematological malignancies in which it usually imparts more aggressive, chemo-refractory, and poor prognosis diseases. TP53 mutations are, though, remarkably rare in chronic myelomonocytic leukemia (CMML): affecting 2.4% of cases in a recent study ( Gurney et al, Leukemia 2023). We found pathogenic TP53 mutations ( TP53MT) in only 1.1% of 640 genotyped UK CMML patients. As expected, TP53MT conferred worse leukemia-free (LFS: median 23 v 28 mo, p=0.031) and overall (OS: 26 v 29 mo, p=0.015) survival compared with TP53WTcases.
The clinical relevance in CMML of TP53 expression, however, remains unexplored. To address this we studied 92 TP53WT CMML patients (discovery cohort) from the National Taiwan University Hospital (Taipei, Taiwan) with available RNA-seq on presentation bone marrow (BM), each with extensive longitudinal clinical, laboratory and outcome data. Validation cohorts comprised 33 RNA-sequenced patients from The Christie (Manchester, UK) and 24 from Hospital Morales Meseguer (Murcia, Spain). For all cohorts, RNA-seq data from healthy controls (HCs) were available.
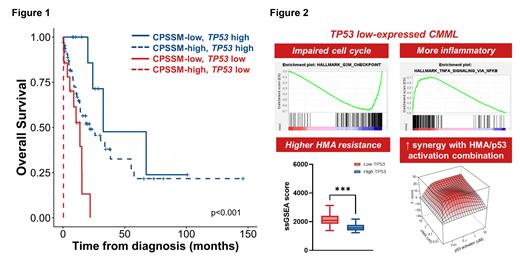
Patients in the discovery cohort were divided into low and high TP53 expression groups, determined by the maximally selected rank statistics. TP53low patients (n=15) displayed significantly lower expression than HCs, while TP53high (n=77) had expression levels comparable to HCs. Thus, although most CMML patients are TP53WT, a subset has down-regulated TP53 expression and potential for altered p53 (and downstream) functions. TP53low and TP53high patients did not differ in clinical features or concurrent genetic alterations, although in all cohorts TP53low patients showed a trend towards poorer response to hypomethylating agents (HMA).
TP53low patients displayed significantly worse outcomes than TP53high patients (LFS 7 v 19 mo, p=0.002; OS 11 v 26 mo, p=0.001). Moreover, TP53 expression further stratified prognosis within different CMML-specific prognostic scoring system (CPSS)-molecular risk groups (Fig 1) . Time-dependent ROC analysis indicated TP53 expression as complementary to current risk stratification systems. In multivariable analysis, low TP53 expression remained prognostically detrimental. Survival analyses were consistent in both validation cohorts.
GSEA and IPA analyses revealed that compared with HCs or TP53high, TP53low cells exhibited depleted expression of p53-dependent pathways, including MYC targets, E2F targets, G2/M checkpoints, and DNA repair. Interestingly, these are all among the most upregulated pathways in TP53MT (v TP53WT) samples across multiple cancers in TCGA data ( Donehower et al, Cell Rep 2019), indicating distinct roles for p53 in regulating these pathways in a TP53WT context, and that the driving biology of TP53low CMML is distinct from (and not functionally equivalent to) that of oncogenic TP53 mutations. TP53low patients demonstrated significantly enhanced TNF-alpha and inflammatory response signals, highlighting other distinctive pathobiology of the TP53low subgroup. In line with our clinical observation, single-sample GSEA showed significant enrichment of HMA resistance signatures in TP53low patients, potentially contributing to the dismal prognosis in this group.
We next investigated whether this could be exploited therapeutically. We treated primary CMML BM cells ex vivo with azacitidine and NSC-207895, an MDMX inhibitor with p53 activating properties, observing clear and substantial synergy for combination therapy in samples from 10 of 11 patients. There was a trend towards inverse correlation between TP53 expression and empirical synergy scores (r=-0.35), suggesting potential for p53 activation to enhance HMA sensitivity in CMML, with broad efficacy, perhaps preferentially in adverse risk TP53low cases.
In summary, we have identified and validated, across three cohorts from three countries, that a subgroup of CMML patients display aberrantly low TP53 expression, associated with HMA resistance and worse survival. Transcriptomic analyses revealed distinctive biology driving this novel TP53low subgroup (Fig 2), mandating further mechanistic study. Importantly we report consistent and substantial synergy for combining pharmacological p53 activation with HMA, presenting a novel targeted approach to improve HMA response for this unmet clinical need.
Disclosures
Hou:Astellas Pharma Ltd: Current Employment. Jerez:Astrazeneca: Research Funding; GILEAD: Research Funding; Novartis: Consultancy; BMS: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal